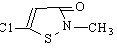LIST:
|
Functional Ingredients |
||||
|
Product Name |
Active Ingredient |
Description |
||
|
INCI |
CAS |
EINECS |
||
|
Sunpu®Allantoin |
Allantoin |
97-59-6 |
202-592-8 |
White crystalline powder. Cosmetic Grade: 98.0~103.0% Pharmaceutic Grade: 98.5~101.0%; conforms to USP and Ph. Eur. |
|
Personal Care Preservatives |
||||
|
Product Name |
Active Ingredient |
Description |
||
|
INCI |
CAS |
EINECS |
||
|
SupGuard® GM-A |
Diazolidinyl Urea |
78491-02-8 |
278-928-2 |
White, fine, free-flowing powder |
|
SupGuard® GM-115 |
Imidazolidinyl Urea |
39236-46-9 |
254-372-6 |
White, fine, free-flowing powder |
|
SupGuard® GM-B |
Diazolidinyl Urea Methylparaben Propylparaben |
78491-02-8 99-76-3 94-13-3 |
278-928-2 202-785-7 202-307-7 |
Colorless to light yellow clear viscous liquid. For formulations with 75% or higher water content |
|
SupGuard® GM-BE |
Diazolidinyl Urea Methylparaben Propylparaben |
78491-02-8 99-76-3 94-13-3 |
278-928-2 202-785-7 202-307-7 |
Colorless to light yellow clear viscous liquid. For emulsion systems with oil phases greater than 25% |
|
SupGuard® GM-P |
Diazolidinyl Urea Iodopropynyl Butylcarbamate |
78491-02-8 55406-53-6 |
278-928-2 259-627-5 |
White, fine, free-flowing powder |
|
SupGuard® GM-BP |
Diazolidinyl Urea Iodopropynyl Butylcarbamate |
78491-02-8 55406-53-6 |
278-928-2 259-627-5 |
Colorless to light yellow clear viscous liquid |
|
SupGuard® GM-C |
Diazolidinyl Urea Methylparaben Iodopropynyl Butylcarbamate |
78491-02-8 99-76-3 55406-53-6 |
278-928-2 202-785-7 259-627-5 |
Colorless to light yellow clear viscous liquid |
|
SupGuard® K15 |
Methylchloroisothiazolinone Methylisothiazolinone |
26172-55-4 2682-20-4 |
247-500-7 220-239-6 |
≥1.5% a.i.. Clear liquid. Magnesium salt stabilized |
|
SupGuard® MIT |
Methylisothiazolinone |
2682-20-4 |
220-239-6 |
≥9.5% a.i.. Clear liquid |
|
SupGuard® G |
DMDM Hydantoin |
6440-58-0 |
229-222-8 |
55% a.i.. Colorless clear liquid |
|
SupGuard® LGP |
DMDM Hydantoin Iodopropynyl Butylcarbamate Methylisothiazolinone |
6440-58-0 55406-53-6 2682-20-4 |
229-222-8 259-627-5 220-239-6 |
Colorless clear liquid |
|
SupGuard® HG |
Sodium Hydroxymethylglycinate |
70161-44-3 |
274-357-8 |
50% a.i.. Colorless to light yellow clear liquid |
|
SupGuard® IPBC-I |
Iodopropynyl Butylcarbamate |
55406-53-6 |
259-627-5 |
≥99% a.i.. White crystalline powder |
|
SupGuard® IPBC-II |
Iodopropynyl Butylcarbamate |
55406-53-6 |
259-627-5 |
6% a.i.. Viscous liquid. Water soluble and especially for high aqueous formulations |
|
SupGuard® IPBC-III |
Phenoxyethanol Iodopropynyl Butylcarbamate |
122-99-6 55406-53-6 |
204-589-7 259-627-5 |
Light yellow clear liquid. Especially for high oil phase formulations and anhydrous systems |
|
SupGuard® PE10 |
Phenoxyethanol |
122-99-6 |
204-589-7 |
≥99% a.i.. Colorless clear liquid |
|
SupGuard® PE51 |
Phenoxyethanol Methylparaben Ethylparaben Propylparaben Butylparaben Isobutylparaben |
122-99-6 99-76-3 120-47-8 94-13-3 94-26-8 4247-02-3 |
204-589-7 202-785-7 204-399-4 202-307-7 202-318-7 224-208-8 |
Colorless to light yellow viscous liquid |
|
SupGuard® BN |
2-Bromo-2-nitropropane-1,3-diol |
52-51-7 |
200-143-0 |
≥99% a.i.. White or almost white crystalline powder |
|
SupGuard® NP-M |
Methylparaben |
99-76-3 |
202-785-7 |
≥99% a.i.. White crystalline powder |
|
SupGuard® NP-E |
Ethylparaben |
120-47-8 |
204-399-4 |
≥99% a.i.. White crystalline powder |
|
SupGuard® NP-P |
Propylparaben |
94-13-3 |
202-307-7 |
≥99% a.i.. White crystalline powder |
|
SupGuard® NP-B |
Butylparaben |
94-26-8 |
202-318-7 |
≥99% a.i.. White or almost white crystalline powder |
|
SupGuard® NP-IB |
Isobutylparaben |
4247-02-3 |
224-208-8 |
≥99% a.i.. White or almost white crystalline powder |
|
SupGuard® NP-IP |
Isopropylparaben |
4191-73-5 |
224-069-3 |
≥99% a.i.. White or almost white crystalline powder |
|
SupGuard® NP-Oil |
Isopropylparaben Isobutylparaben Butylparaben |
4191-73-5 4247-02-3 94-26-8 |
224-069-3 224-208-8 202-318-7 |
≥97% total parabens. Solvent-free, light yellow, clear stable viscous liquid. Especially for high oil phase formulations and anhydrous systems |
|
SupGuard® BC |
Benzethonium Chloride |
121-54-0 |
204-479-9 |
≥97% a.i.. White or almost white crystalline powder |
|
SupGuard® BC200 |
Benzethonium Chloride |
121-54-0 |
204-479-9 |
Synergistic formulation. Clear viscous liquid. Especially for hygienic wipes. |
|
Triclosan-I |
Triclosan |
3380-34-5 |
222-182-2 |
≥99% a.i.. White crystalline powder |
|
Triclosan-II |
Triclosan |
3380-34-5 |
222-182-2 |
10% a.i.. Viscous liquid. Water soluble and especially for high aqueous formulations |
|
Industrial Biocides |
||||
|
Product Name |
Active Ingredient |
Description |
||
|
INCI |
CAS |
EINECS |
||
|
SupBio® MP31 |
Formulation |
―― |
―― |
Special for metalworking fluids. Especially for water-based systems |
|
SupBio® K15 |
Methylchloroisothiazolinone Methylisothiazolinone |
26172-55-4 2682-20-4 |
247-500-7 220-239-6 |
≥1.5% a.i.. Clear Liquid. Copper salt stabilized |
|
SupBio® W140 |
Methylchloroisothiazolinone Methylisothiazolinone |
26172-55-4 2682-20-4 |
247-500-7 220-239-6 |
≥14.0% a.i.. Clear Liquid. Magnesium salt stabilized |
|
Functional Wipe Concentrates |
||||
|
Product Name |
Active Ingredient |
Description |
||
|
INCI |
CAS |
EINECS |
||
|
SupClean®BW |
Formulation |
―― |
―― |
Special for glass. Easy to remove grease, dirt, stains and finger marks. Quick drying, streak-free and anti-fog |
Sunpu® Allantoin
DESCRIPTION
|
INCI: |
Allantoin |
|
CAS: |
97-59-6 |
|
EINECS: |
202-592-8 |
|
Molecular Formula: |
C4H6N4O3 |
|
Molecular Weight: |
158.12 |
Structural Formula: 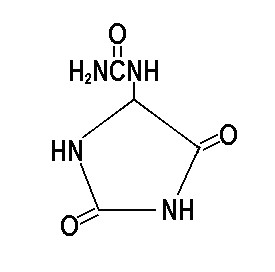
|
Synonyms: |
5-Ureidohydantoin; Glyoxylic acid diureide; 2,5-Dioxo-4-imidazolidinyl urea |
SPECIFICATIONS (Not Necessarily)
|
Properties |
Cosmetic Grade |
Pharmaceutic Grade* |
|
Appearance |
White crystalline powder |
White crystalline powder |
|
Odor |
Odorless |
Odorless |
|
Identification (a) IR Spectrum |
- |
Conforms |
|
(b) TLC Test |
- |
Conforms |
|
Content |
98.0-103.0% |
98.5-101.0% |
|
Melting Point (with decomposition) |
223℃ min. |
225℃ min. |
|
Loss on Drying |
0.5% max. |
0.1% max. |
|
Residue on Ignition |
0.5% max. |
0.1% max. |
|
pH (0.5% in water, 25℃) |
4.0-6.0 |
- |
|
Acidity or Alkalinity |
- |
Conforms |
|
Optical Rotation |
- |
Conforms |
|
Reducing Substances |
- |
Conforms |
|
Related Substances |
- |
Conforms |
|
As |
0.0002% max. |
0.0002% max. |
|
Pb |
0.001% max. |
0.001% max. |
* Conforms to USP and Ph. Eur.
PACKAGING
Standard packaging includes the following:
1.0kg/bottle; 10bottles/box
20 kg net weight cardboard drum inside plastic bag sealed
Other package sizes are available upon request.
STORAGE
Store in a well-ventilated, cool, dry area, out of direct sunlight. Keep containers tightly closed when not in use.
PERIOD OF VALIDITY
24 months
PROPERTIES
Solubility
Solubility in water increases obviously with increasing temperature (5.7g/L at 25℃;11g/L at 40℃;40g/L at 75℃).
Slightly soluble in propylene glycol (3g/L at 25℃).
Very slightly soluble in ethanol (0.4g/L at 25℃) and glycerin (0.15g/L at 25℃).
Biological Activity
Allantoin promotes wound healing, speeds up cell regeneration, and has a keratolytic effect. It produces its desirable effects by promoting and speeding up the healthy, natural processes of the body. It helps the skin to help itself.
Toxicological Data
|
Acute oral toxicity LD50 (rats): |
>5000mg/kg |
|
Eye irritation (rabbits): |
No irritation |
|
Skin irritation: |
Non-irritating at normal use levels |
REGULATORY STATUS
|
In the USA: |
Not restricted in any way if used as a cosmetic ingredient. Classified by the FDA as a Category I (safe and effective), OTC drug (skin protectant) if used as an active ingredient. |
|
In the EU: (76/768/EEC) |
No direct regulations. No limitations; permitted for cosmetic use. |
|
In Japan: |
Not specifically regulated according to the Standards for Cosmetics, enacted by the Ministry of Health, Labour and Welfare. |
APPLICATIONS
In pharmaceuticals and dermatology, allantoin is used in the treatment of ulcers, slow-healing wounds and burns. Addition of it to wound ointments and healing compositions promotes wound cleansing and healing. Allantoin has been classified by the US FDA OTC Topical Analgesic Review Panel as a Category I active ingredient (skin protectant), at use-levels of 0.5-2.0%. The FDA approved applications include minor cuts, scrapes, burns, sunburn, fever blisters, diaper rash and chafed, chapped, cracked or wind burned skin and lips.
In cosmetics, allantoin is used in numerous preparations, where it enhances cell renewal, skin soothing, skin moisturizing, cleansing and healing action. In addition, it has the advantages of being non-toxic, non-irritating and effective in low concentrations. Allantoin promotes cell regeneration in skin exposed to and weakened by environmental factors, and helps to promote and maintain the health of intact skin on the body and face. Addition of it to hand lotions and hand creams helps eliminate rough, chapped skin, and leaves hands smooth and soft. In hair care, because of its extraordinarily good keratolytic properties, allantoin is a valuable additive in various products such as shampoos and hair gels, especially for the treatment of dandruff. Allantoin has been incorporated into various lotions and creams, lipsticks, aftershave balms, suntan products, bath foams, baby products and various aerosol preparations. It has also been used in oral hygiene products such as toothpaste and mouthwash. Its good compatibility with most relevant materials also allows it to be easily incorporated into existing formulations.
The recommended use level in cosmetics is 0.1-0.5%.
The information in this document corresponds to the present state of our knowledge and is intended to describe our products and their possible applications. No warranty is either expressed or implied. The values given are recommended guides. All responsibility for determining the most effective use-level remains with the final product manufacturer.
SupGuard GM-115 (Imidazolidinyl Urea )
INCI Adopted Name:Imidazolidinyl Urea
CAS Registry No.:39236-46-9
Molecular Formula:C11H16N8O8
Molecular Weight:388.30
Structural Formula:
Synonyms:N,N"-methylenebis[N'-(3-hydroxymethyl-2,5-dioxo-4-imidazolidinyl)] Urea; 3,3'-Bis(1-hydroxymethyl-2,5-dioxoimidazolidin-4-yl)-1,1'- methylenediurea
SPECIFICATIONS (Not Necessarily)
AppearanceWhite, fine, free-flowing powder
OdorOdorless or slight characteristic odor
Nitrogen26.0-28.0%
Loss on Drying3.0% max.
Residue on Ignition3.0% max.
pH (1% in water)6.0-7.5
As0.001% max.
Pb0.004% max.
PACKAGING
Standard packaging includes the following:
1.0kg/bottle; 10bottles/box
20 kg net weight cardboard drum inside plastic bag sealed Other package sizes are available upon request.
STORAGE
Store in a well-ventilated, cool, dry area, out of direct sunlight. Keep containers tightly closed when not in use.
PERIOD OF VALIDITY
24 months
PROPERTIES
Solubility
Very soluble in water; freely soluble in propylene glycol and glycerin;
slightly soluble in methanol and ethanol; not soluble in mineral oil.
Antimicrobial Activity
SupGuard GM-115 is very effective against Gram-negative bacteria, including Pseudomonas aeruginosa. It acts synergistically with other preservatives. In combination with Parabens and/or Iodopropynyl Butylcarbamate (IPBC), SupGuard GM-115 provides a complete antimicrobial preservative system that is active against bacteria, yeast and mold.
Toxicological Data
Acute oral toxicity LD50 rats):5000mg/kg Acute dermal toxicity LD50 (rabbits):>8000mg/kg
Eye irritation (rabbits):Non-irritating at 5% aqueous solution
Primary skin irritation (rabbits):Non-irritating at 5% aqueous solution
Applications
The active ingredient of SupGuard GM-115, Imidazolidinyl Urea, is permanently listed by the EU at levels up to 0.6% without restrictions. It is one of the most widely used cosmetic preservatives in the world.
SupGuard GM-115 is an effective antibacterial preservative in a wide range of liquid and powder type cosmetic products. It is compatible with essentially all cosmetic ingredients, such as surfactants, proteins, and other special ingredients. As a result of its high solubility in water,
SupGuard GM-115 can be successfully incorporated into cold mix formulations. It is safe and effective for both leave-on and rinse-off products. The recommended use level is 0.2-0.4%.
The information in this publication corresponds to the present state of our knowledge and is intended to describe our products and their possible applications.
No warranty is either expressed or implied. Every cosmetic formulation requires a tailormade preservative system to meet its special needs.
Every developed or modified product should be challenge tested to assure preservative efficacy.
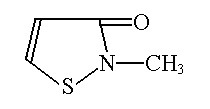
SupGuard GM-A(Diazolidinyl Urea )
INCI Adopted Name:Diazolidinyl Urea
CAS Registry No.:78491-02-8
Molecular Formula:C8H14N4O7
Molecular Weight:278.22
Structural Formula:
Synonyms:N-(1,3-Bis(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl)-N,N'- bis(hydroxymethyl)urea; N-(Hydroxymethyl)-N-(1,3-dihydroxymethyl-2,5-dioxo-4-imidazolidinyl)- N'-(hydroxymethyl)urea
SPECIFICATIONS (Not Necessarily)
AppearanceWhite, fine, free-flowing powder
OdorOdorless or slight characteristic odor
Nitrogen19.0-21.0%
Loss on Drying3.0% max.
Residue on Ignition3.0% max.
pH (1% in water)6.0-8.0
As0.001% max.
Pb0.004% max.
PACKAGING
Standard packaging includes the following:
1.0kg/bottle; 10bottles/box
20 kg net weight cardboard drum inside plastic bag sealed
Other package sizes are available upon request.
STORAGE
Store in a well-ventilated, cool, dry area, out of direct sunlight. Keep containers tightly closed when not in use.
PERIOD OF VALIDITY
24 months
PROPERTIES
Solubility
Very soluble in water; freely soluble in propylene glycol and glycerin;
slightly soluble in methanol and ethanol; not soluble in mineral oil.
Antimicrobial Activity
SupGuard GM-A provides a broad spectrum of antibacterial activity against Gram-positive and Gram-negative bacteria, including Pseudomonas species; and some protection against yeast and mold.
Toxicological Data
Acute oral toxicity LD50 (mice):3200mg/kg
Eye irritation (rabbits):Non-irritating at 3% aqueous solution
Primary skin irritation (rabbits):Non-irritating at 3% aqueous solution
Applications
The active ingredient of SupGuard GM-A, Diazolidinyl Urea, is permanently listed by the EU at levels up to 0.5% without restrictions.
SupGuard GM-A is safe for both leave-on and rinse-off products and provides effective protection at low concentration levels. It is typically used at concentrations from 0.1-0.3%.
SupGuard GM-A is compatible with virtually all cosmetic ingredients. It is not inactivated by emulsifiers, proteins or other ingredients that affect other preservative materials.
SupGuard GM-A is an excellent preservative for shampoos and hair conditioners. It is synergistic with other preservative materials, especially parabens and/or Iodopropynyl Butylcarbamate (IPBC). This combination is a complete cosmetic preservative systems that is effective at protecting well formulated products from bacterial, yeast and mold contamination during use. The activity against yeast and mold does not decrease while SupGuard GM-A is used in combination with parabens even when paraben effectiveness has been diminished by interaction with nonionics or proteins, or migration into the oil phase.
The information in this publication corresponds to the present state of our knowledge and is intended to describe our products and their possible applications.
No warranty is either expressed or implied. Every cosmetic formulation requires a tailormade preservative system to meet its special needs.
Every developed or modified product should be challenge tested to assure preservative efficacy.
SupGuard K15
SupGuard K15 is a liquid preservative system with the following active ingredients:
|
INCI Adopted Name |
Structural Formula |
CAS Registry No. |
|
Methylchloroisothiazolinone |
|
26172-55-4 |
|
Methylisothiazolinone |
|
2682-20-4 |
Like Product Name: Kathon CG
SPECIFICATIONS (Not Necessarily)
|
Appearance |
Light amber clear liquid |
|
Odor |
Characteristically mild |
|
Active Ingredients |
1.5% min. |
|
Magnesium Salts (nitrate and chloride) |
21-24% |
|
Specific Gravity(20℃) |
1.19-1.21 |
|
pH |
2.5-4.0 |
|
As |
0.001% max. |
|
Pb |
0.004% max. |
PACKAGING
Standard packaging includes the following:
1.0kg/bottle; 10bottles/box
20kg net weight plastic pail
220kg net weight plastic drum
Other package sizes are available upon request.
STORAGE
Store in a well-ventilated, cool area, out of direct sunlight. Keep containers tightly closed when not in use.
PERIOD OF VALIDITY
12 months since the date of manufacture
PROPERTIES
Solubility
Completely soluble in water; soluble in lower alcohols and glycols.
Antimicrobial Activity
SupGuard K15 has extremely broad spectrum activity against Gram-positive and Gram-negative bacteria, molds and yeasts. It can be used to make high performance bactericides.
Toxicological Data
|
Acute oral toxicity LD50 (mice): |
3160mg/kg (female), 3480mg/kg (male) |
|
Acute dermal toxicity LD50 (rabbits): |
﹥5000mg/kg |
|
Eye irritation (rabbits): |
Non-irritating at 0.5% aqueous solution |
|
Primary skin irritation (rabbits): |
Non-irritating at 0.5% aqueous solution |
Applications in Cosmetics
The active ingredients of SupGuard K15 are globally approved as a safe, effective preservative for rinse-off products such as shampoos and conditioners. It is listed in the USA and the EU respectively as an approved cosmetic preservative at levels up to 0.0015% of active ingredients.
SupGuard K15 does not bioaccumulate. It is biodegradable and degrades rapidly to non-toxic, non-persistent substances in the environment. SupGuard K15 is effective and stable up to pH8. It is generally compatible with most components of formulations. However, the presence of a few agents such as thiols, mercaptans, secondary amines, sulfides and other nucleophiles will cause degradation of the active ingredients. Conditions of temperatures in excess of 50℃ for long periods of time and pH above 8 will lead to loss of activity.
The maximum recommended use level is 0.1% by weight of product as supplied in rinse-off products and 0.05% in leave-on products.
SupGuard® GM-B
SupGuard® GM-B is a liquid preservative system with the following composition:
|
INCI |
Structural Formula |
CAS |
EINECS |
|
Diazolidinyl Urea |
|
78491-02-8 |
278-928-2 |
|
Methylparaben |
|
99-76-3 |
202-785-7 |
|
Propylparaben |
|
94-13-3 |
202-307-7 |
|
Propylene Glycol |
|
57-55-6 |
200-338-0 |
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
Colorless to light yellow clear viscous liquid |
|
Odor |
Characteristically mild |
|
Nitrogen |
5.4-6.8% |
|
Methylparaben |
11% |
|
Propylparaben |
3% |
|
Specific Gravity(20℃) |
1.16-1.19 |
|
Residue on Ignition |
0.8% max. |
|
Total Solids |
42.5-45.5% |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
SupGuard® GM-P
SupGuard® GM-BP is a liquid preservative system with the following composition:
|
INCI |
Structural Formula |
CAS |
EINECS |
|
Diazolidinyl Urea |
|
78491-02-8 |
278-928-2 |
|
Iodopropynyl Butylcarbamate |
|
55406-53-6 |
259-627-5 |
|
Propylene Glycol |
|
57-55-6 |
200-338-0 |
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
Colorless to slight yellow clear viscous liquid |
|
Odor |
Characteristically mild |
|
Color (Hazen) |
50 max. |
|
Nitrogen |
7.5-8.5% |
|
IPBC |
0.35-0.55% |
|
Specific Gravity(20℃) |
1.15-1.25 |
|
Residue on Ignition |
1.5% max. |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
SupGuard® GM-P
SupGuard® GM-P is a broad spectrum, non-paraben preservative system with the following composition:
|
INCI |
Structural Formula |
CAS |
EINECS |
|
Diazolidinyl Urea |
|
78491-02-8 |
278-928-2 |
|
Iodopropynyl Butylcarbamate |
|
55406-53-6 |
259-627-5 |
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
White, fine, free-flowing powder |
|
Odor |
Characteristically mild |
|
Nitrogen |
19.0-21.0% |
|
IPBC |
0.75-1.25% |
|
Loss on Drying |
3.0% max. |
|
Residue on Ignition |
3.0% max. |
|
pH (1% in water) |
6.0-7.5 |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
SupGuard® PE51
SupGuard® PE51 is a liquid preservative system with the following active ingredients:
|
INCI |
Structural Formula |
CAS |
EINECS |
|
Phenoxyethanol |
|
122-99-6 |
204-589-7 |
|
Methylparaben |
|
99-76-3 |
202-785-7 |
|
Ethylparaben |
|
120-47-8 |
204-399-4 |
|
Propylparaben |
|
94-13-3 |
202-307-7 |
|
Butylparaben |
|
94-26-8 |
202-318-7 |
|
Isobutylparaben |
|
4247-02-3 |
224-208-8 |
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
Colorless to light yellow, clear liquid |
|
Odor |
Characteristically mild |
|
Specific Gravity(25℃) |
1.11-1.14 |
|
Refractive Index |
1.538-1.545 |
|
Total Parabens |
28-32% |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
SupGuard G(DMDM Hydantoin)
|
INCI Adopted Name: |
DMDM Hydantoin |
|
CAS Registry No.: |
6440-58-0 |
|
Molecular Formula: |
C7H12N2O4 |
|
Molecular Weight: |
188.18 |
Structural Formula: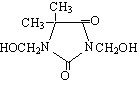
|
Like Product Name: |
Glydant |
SPECIFICATIONS (Not Necessarily)
|
Appearance |
Colorless to light yellow clear liquid |
|
Odor |
Odorless or characteristically mild |
|
Nitrogen |
7.8-8.6% min. |
|
Specific Gravity(25℃) |
1.14-1.17 |
|
pH |
5.0-7.0 |
|
Total Solids |
54-56% |
|
As |
0.001% max. |
|
Pb |
0.004% max. |
PACKAGING
Standard packaging includes the following:
1.0kg/bottle; 10bottles/box
20kg net weight plastic pail
220kg net weight plastic drum
Other package sizes are available upon request.
STORAGE
Avoid freezing. Store at or near room temperature, out of direct sunlight. Keep containers tightly closed when not in use.
Storage at temperatures below 10°C for a long period of time, may cause formation of crystals, requires thawing and mixing prior to use, with no detrimental effect to product quality or efficacy.
PERIOD OF VALIDITY
24 months since the date of manufacture
PROPERTIES
Solubility
Very soluble in water; soluble in lower alcohols and glycols.
Antimicrobial Activity
SupGuard G is very effective against Gram-positive and Gram-negative bacteria, also provides some protection against yeasts and molds.
Toxicological Data
|
Acute oral toxicity LD50 (mice): |
﹥2000mg/kg |
|
Eye irritation (rabbits): |
Non-irritating at 0.6% aqueous solution |
|
Primary skin irritation (rabbits): |
Non-irritating at 0.6% aqueous solution |
Applications in Cosmetics
The active ingredient of SupGuard G, DMDM Hydantoin, is permitted for use in cosmetic and personal care products in the USA and the EU to a maximum concentration of 0.6%.
SupGuard G is compatible with essentially all cosmetic ingredients, such as surfactants, emulsifiers, proteins, aloe and amines. SupGuard G is an excellent preservative for both leave-on and rinse-off products such as shampoos, creams, lotions, bubble baths, rinses, wipes and towelettes. It is easy to handle, very stable and cost-effective. The recommended use level is 0.1~0.6% by weight of product as supplied.
SupGuard G exhibits an excellent synergistic preservative effect in combination with Iodopropynyl Butylcarbamate (IPBC), Parabens and Isothiazolinones. It is stable over a wide range of pH and temperature conditions. It does not discolor product when combined with additives or by changes in pH. It can be added at a wide range of temperatures from ambient to elevated 80℃.
SupGuard® HG
|
INCI: |
Sodium Hydroxymethylglycinate |
|
CAS: |
70161-44-3 |
|
EINECS: |
274-357-8 |
|
Molecular Formula: |
C3H6NO3Na |
|
Molecular Weight: |
127.07 |
|
Structural Formula: |
|
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
Colorless to light yellow clear liquid |
|
Odor |
Characteristically mild |
|
Nitrogen |
5.4-6.0% |
|
Specific Gravity(25℃) |
1.27-1.30 |
|
pH |
10.0-12.0 |
|
Total Solids |
49-52% |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
SupGuard® NP-M
|
INCI: |
Methylparaben |
|
CAS: |
99-76-3 |
|
EINECS: |
202-785-7 |
|
Molecular Formula: |
C8H8O3 |
|
Molecular Weight: |
152.15 |
|
Structural Formula: |
|
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
White crystalline powder |
|
Odor |
Odorless or characteristically mild |
|
Content |
99.0% min. |
|
Melting Point |
125-128℃ |
|
Loss on Drying |
0.5% max. |
|
Residue on Ignition |
0.1% max. |
|
Acidity |
Pass |
|
Sulfate (based on SO42-) |
0.024% max. |
|
p-Hydroxybenzoic Acid and Salicylic Acid |
Pass |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
*Conforms to USP29 and Ph. Eur. 6.0
SupGuard® IPBC-II
|
INCI: |
Iodopropynyl Butylcarbamate |
|
CAS: |
55406-53-6 |
|
EINECS: |
259-627-5 |
|
Molecular Formula: |
C8H12INO2 |
|
Molecular Weight: |
281.09 |
|
Structural Formula: |
|
SupGuard® IPBC-II is one of the most advanced preservatives available to date. This is a newly patented (ZL200410090830.4) liquid product of Iodopropynyl Butylcarbamate (IPBC). It is water soluble and provides for easy addition of IPBC to a wider range of personal care formulations, especially high aqueous formulations, than ever before.
TYPICAL PROPERTIES (Not to be considered product specifications)
|
Appearance |
Colorless to light yellow, clear viscous liquid at 25℃ |
|
Odor |
Characteristically mild |
|
Active Ingredient |
5.8-6.2% |
|
Specific Gravity(25℃) |
1.10-1.13 |
|
As |
0.0003% max. |
|
Pb |
0.001% max. |
|
Stability |
Soluble in ethanol, propylene glycol, polyethylene glycol and water. |
SupGuard BN(Bronopol)
DESCRIPTION
|
INCI Adopted Name: |
2-Bromo-2-Nitropropane-1,3-Diol |
|
CAS Registry No.: |
52-51-7 |
|
Molecular Formula: |
C3H6BrNO4 |
|
Molecular Weight: |
199.99 |
|
Like Product Name: |
Bioban Myacide |
Structural Formula: 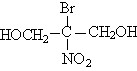
SPECIFICATIONS (Not Necessarily)
|
Appearance |
White or almost white crystalline powder |
|
Odor |
Odorless or almost odorless |
|
Content |
99.0% min. |
|
Melting Point |
125℃ min. |
|
Free Bromine |
0.3% max. |
|
Loss on Drying |
0.3% max. |
|
Residue on Ignition |
0.3% max. |
|
As |
0.001% max. |
|
Pb |
0.004% max. |
PACKAGING
Standard packaging includes the following:
0.5kg/bottle; 20bottles/box
20 kg net weight cardboard drum inside plastic bag sealed
Other package sizes are available upon request.
STORAGE
Store in a well-ventilated, cool, dry area, out of direct sunlight.
Keep containers tightly closed when not in use.
PERIOD OF VALIDITY
24 months since the date of manufacture
PROPERTIES
Solubility
Freely soluble in water and ethanol; slightly soluble in glycerin and liquid paraffin.
Antimicrobial Activity
SupGuard BN has extremely broad spectrum activity against Gram-positive and Gram-negative bacteria,
yeasts and molds. It is particularly effective against Pseudomonas species.
Toxicological Data
|
Acute oral toxicity LD50 (mice): |
266mg/kg(male), 222mg/kg (female) |
|
Eye irritation (rabbits): |
Slightly irritating at 0.5% aqueous solution; non-irritating at maximum use concentration of 0.1% |
|
Primary skin irritation (rabbits): |
Slightly irritating at 0.5% aqueous solution; non-irritating at maximum use concentration of 0.1% |
Applications in Cosmetics
The active ingredient of SupGuard BN is 2-Bromo-2-Nitropropane-1,3-Diol or Bronopol. It is listed in the USA and the EU respectively as an approved cosmetic preservative at levels up to 0.1%.
SupGuard BN is an excellent preservative for both leave-on and rinse-off products such as creams, lotions, shampoos, rinses, wipes and towelettes. The recommended use level is 0.02~0.05%.
SupGuard BN is compatible with most cosmetic ingredients such as surfactants, emulsifiers, proteins and natural extracts. It is synergistic with other preservatives such as isothiazolinones, parabens and Iodopropynyl Butylcarbamate (IPBC). Its optimum pH range of use is 4~8. It should be added as the last ingredient and at the lowest temperature (≤50℃) if possible.
Conditions of high temperatures, alkaline and sunlight will cause degradation of the active ingredient. The presence of a few agents such as sulfhydryl compounds, sulfite, hyposulfite, aluminous and ferreous materials will lead to loss of activity. The use of secondary amines should be avoided in formulations.